Four Fold Serial Dilutions
A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. In serial dilutions, you multiply the dilution factors for each step. The dilution factor or the dilution is the initial volume divided by the final volume.
#DF = V_i/V_f# For example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution, #DF = V_i/V_f# = #(1'mL')/(10'mL') = 1/10#. This is a 1:10 dilution. Example 1 What is the dilution factor if you add 0.2 mL of a stock solution to 3.8 mL of diluent? #V_f# = 0.2 mL + 3.8 mL = 4.0 mL #DF = V_i/V_f# = #(0.2'mL')/(4.0'mL') = 1/20#. This is a 1:20 dilution. Example 2 If you did the above dilution four times, what would be the final dilution factor? Solution 2 Remember that serial dilutions are always made by taking a set quantity of the initial dilution and adding it successively to tubes with the same volume. Download driver 101 102 key or microsoft natural ps 2 keyboard.
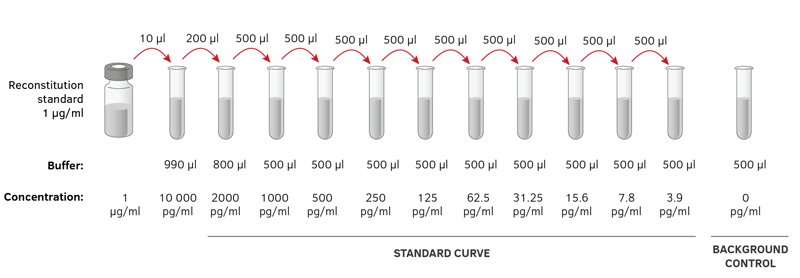
So you multiply each successive dilution by the dilution factor. You would transfer 0.2 mL from Tube 1 to 3.8 mL of diluent in Tube 2 and mix.
Then transfer 0.2 mL from Tube 2 to 3.8 mL of diluent in Tube 3 and mix. Repeat the process until you have four tubes. The dilution factor after four dilutions is #DF = 1/20 × 1/20 × 1/20 × 1/20 = 1/160000# = 1:160 000 If the concentration of the original stock solution was 100 µg/µL, the concentration in Tube 4 would be 100 µg/µL × #1/160000# = 6.25 × 10⁻⁴ µg/µL Hope this helps.
Jun 29, 2018 How to Do Serial Dilutions. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. It’s helpful to label all of your tubes before you begin so you don’t get confused once you begin with.